
![]()
Serial column
10/2022
[Battery materials] Vol.2 Binder for lithium-ion batteries
Takanori Suzuki
Suzuki Material Technology and Consulting Co., Ltd.
[Serial column] In this column, the market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the second column of the series is “Binder for lithium-ion batteries.”
【Serial column : Battery materials】
>Vol.01 Lithium-ion batteries and fluoromaterials
Vol.02 Binder for lithium-ion batteries
1. What is a binder?
All batteries - not only lithium-ion batteries - contain materials that serve as an anode and cathode. These materials are called active materials. In lithium-ion batteries, lithium-containing metal oxides are generally used as the cathode and graphite as the anode. Active materials are in the form of a powder, and binders are "glue" used to hold them together and fix them on a metal foil called a current collector foil (cathode: aluminum foil, anode: copper foil).
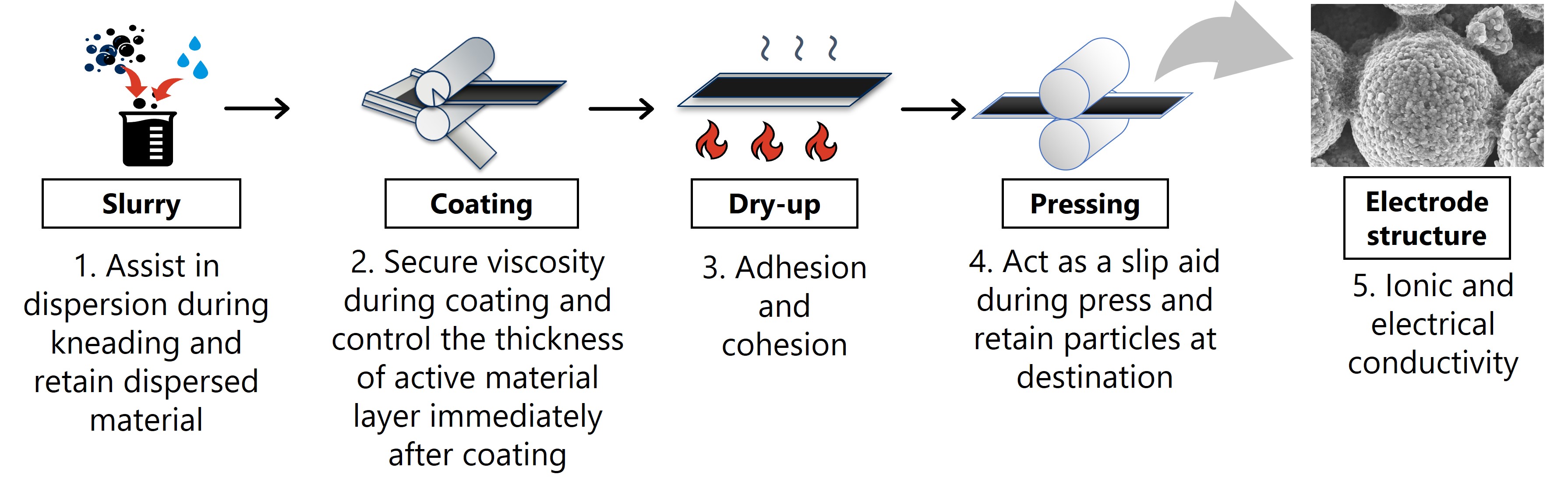
In addition to the role as "glue", binders are required to have many roles including the following.
- 1-1. Assist in dispersion during kneading and retain dispersed material
- 1-2. Secure viscosity during coating and control the thickness of active material layer immediately after coating
- 1-3. Adhesion and cohesion
- 1-4. Act as a slip aid during press and retain particles at destination
- 1-5. Ionic and electrical conductivity
Fig.1 Roles of a binder in the electrode manufacturing process
1-1. Assist in dispersion during kneading and retain dispersed material
In the kneading process, it is necessary to disperse solid components such as active materials and conductive agents in a solvent, and to retain them until drying is completed. A binder is dissolved in a solvent to give a proper viscosity and contributes to the dispersion and retention of active materials and conductive agents. Polymer materials are suitable for this purpose because they dissolve in solvents and increase viscosity. In addition, the increased viscosity enhances the shear force between the solution (solvent + polymer) and the solid content during kneading, thereby helping to improve the dispersibility of the solid content. Furthermore, the increased viscosity of the solvent reduces the sedimentation rate of the dispersed solid, allowing for a longer pot life of the slurry.
1-2. Secure viscosity during coating and control the thickness of active material layer immediately after coating
In the coating process, the slurry produced in kneading is thinly coated on the current collector foil. The thickness and dispersion state of the slurry in this process determine important conditions such as the thickness, density, electrode resistance, and porosity of the electrodes after drying and pressing, which directly affect the performance of batteries. The role of a binder is to provide viscoelasticity that enables a uniform film thickness. The slurry of batteries must have a "flowing" property when it is left standing and it needs to cause a uniform "flow" until the moment of coating when the shear rate is large and to stop the flow and move almost immediately after coating when the shear rate becomes almost zero. In other words, the slurry is required to show a contradictory flow performance depending on the shear rate. In general, high-concentration solutions of polymers have such performance by nature, and what determines the state of this performance are the shape of molecular chains, molecular weight, and branching state of the elements constituting the molecules.
1-3. Adhesion and cohesion
As mentioned above, a binder is a glue. The role of the glue is to bind the active material, conductive agent, and current collector foil together. The action is the synthetic force of "adhesion", the force acting on the interface, and "cohesion", the force connecting two materials by the strength of the materials itself. If these two forces are not in good balance, the binder does not act properly as a glue.
Adhesion force is mainly associated with a covalent bonding force, electrical bonding (hydrogen bonding) force, van der Waals force, and anchoring effect. Cohesion force is largely affected by mechanical strength, hardness, and elongation of a material itself. Common polymers often have such forces, which alone provide essential functions as a binder, but they may be suitable or unsuitable for each material depending on the conditions in which they are used.
1-4. Act as a slip aid during press and retain particles at destination
Pressing imparts smoothness to electrodes, forms a uniform film thickness, and increases density. The major change that occurs in pressing is the migration of active material particles. The density is not easily increased by applying pressure to the powder of active material. This is because the entanglement between particles prevents them from moving. The addition of a binder prevents the particles from getting entangled and allows them to pack (move) to achieve a higher density. A binder must change its form to aid particle movement and retain the particles in place after the pressing pressure is released. When a resin is pulled, the stress increases according to the elongation, and when the stress exceeds the yield point, the resin will not return to its original length and stays there even if the pulling is stopped (plastic deformation). A binder such as rubber does not show this yield point and has a strong tendency to return to the original position when the stress is removed (springs back). Therefore, the ability to hold the particles at the destination is important.
1-5. Ionic and electrical conductivity
There are no great expectations for the electrical conductivity of a binder for the anode because graphite is mainly used there, but it is essential for the cathode. However, since a binder is a resin material and not a conductor, a conductive agent that works together with the binder and forms a network is added to impart electrical conductivity to the cathode electrode.
Further, if the electrolyte can be appropriately made to swell in the binder resin, a lithium-ion conductivity is developed through the swollen electrolyte, and so the interaction between the binder and the electrolyte is important. Therefore, a material with a proper electrolyte-swelling property is needed. Of course, if the binder itself has ion conductivity, the electrolyte solution can be eliminated, suggesting the possibility of using the binder as a solid electrolyte.
2. Why fluoropolymer (PVdF)?
Today, almost 100% of the cathodes of lithium-ion batteries use PVdF binders. PVdF resin, a polymer material, has the following properties in addition to its advantage as a binder due to the inherent properties of polymers. There are few materials, other than PVdF, that have these properties in a good balance, which is considered to be why it has been used for many years as a standard binder for a cathode.
Fig.2 Properties of fluoropolymer(PVdF) binder
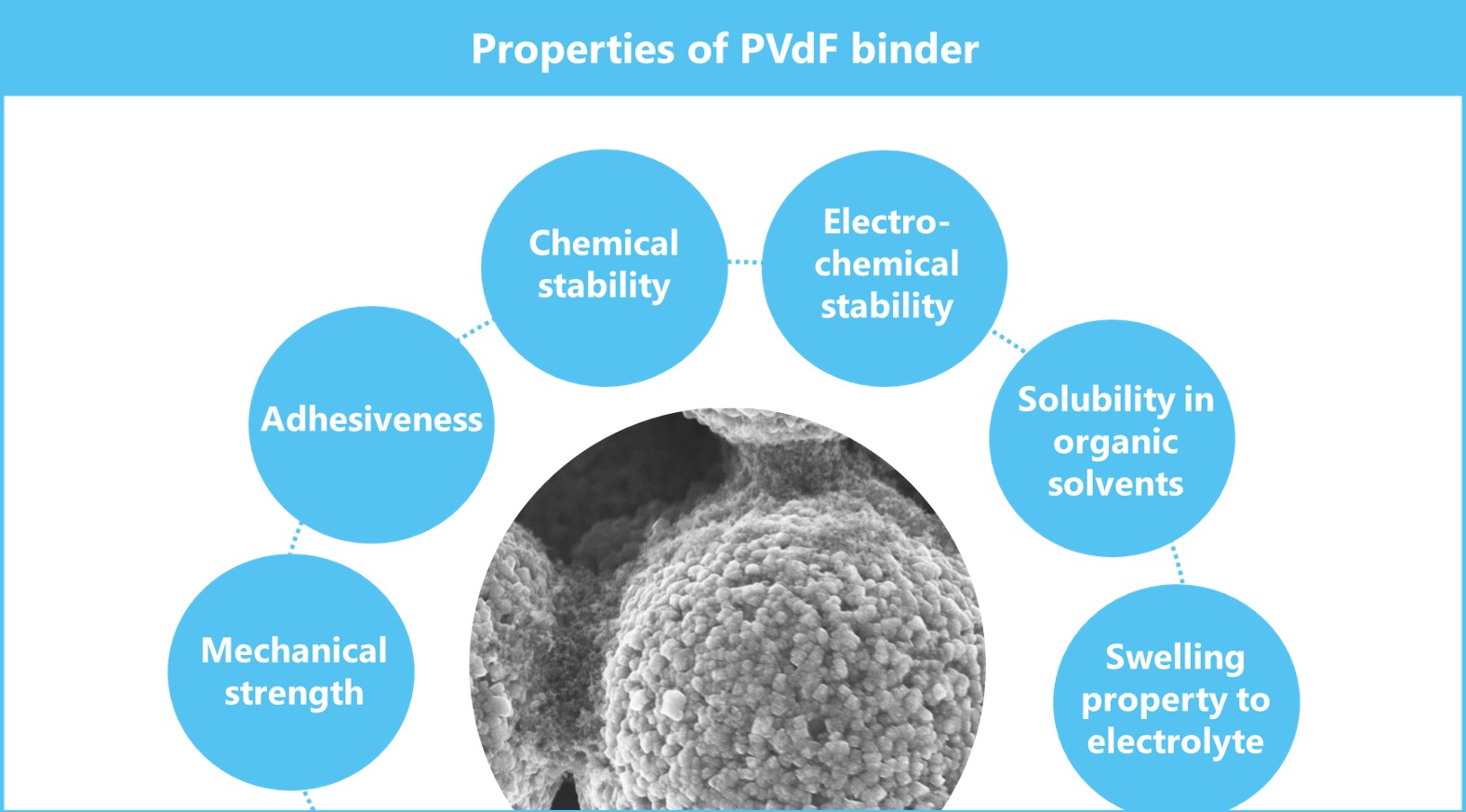
2-1. Mechanical strength
PVdF resin has relatively high strength compared with other fluororesins. As explained earlier, the main contributor to cohesion is the degree of mechanical strength. In addition, PVdF has a molecular weight above a certain level, and it shows a tensile property having a yield point (S-S curve). This feature imparts the performance as a binder of slipping and retaining active materials during pressing.
- 2-2. Adhesiveness
PVdF is a unique fluororesin that shows adhesiveness to metal. This is attributed to its molecular structure in which fluorine, an electron withdrawing group, and hydrogen, an electron emitting group, exist alternately. It is said that the large intramolecular polarization causes electrical polarization, which contributes to adhesion. It is a great advantage as a binder to have an adhesive property despite being a fluororesin and to have excellent mechanical strength at the same time.
- 2-3. Chemical stability
PVdF, a type of fluororesin, is chemically stable by nature. It generally shows high stability to acids, water, organic solvents, oils and fats, and other chemicals. This is attributed to the strength of the C-F bonds, in which fluorine does not come off easily and is less likely to cause chemical changes. It is advantageous for the binders used in carbonate-based organic electrolytes to have low reactivity and solubility in organic solvents.
- 2-4. Electrochemical stability
Current lithium-ion batteries are required to have an oxidation resistance above 4.6 V at the cathode and reduction resistance of up to around 0 V (against the metal potential of Li) at the anode. PVdF has both oxidation and reduction resistance properties satisfying these levels.
- 2-5. Solubility in organic solvents
While general fluororesins are insoluble in organic solvents, PVdF is soluble in certain polar organic solvents such as NMP. Its solubility in certain solvents makes it possible to coat things with it, and this has become the mainstream of the manufacturing process. It has an excellent balance of affinity to the carbonate electrolyte used in lithium-ion batteries to the extent of swelling and non-solubility while exhibiting moderate ion conductivity.
- 2-6. Swelling property to electrolyte
PVdF binders, as described above, swell with general carbonate electrolytes and allow lithium-ions to pass through. Therefore, the ion diffusion resistance inside a battery is lower than that of resin binders which do not allow swelling, leading to a property in which the rate performance of the battery is less likely to drop compared with other resins. If the amount of swelling of an electrolyte is very small, the binder becomes a large resistive component in a battery. The appropriate degree of swelling as a binder is considered to be about 20 to 40%.
3. Daikin’s binder
Daikin has improved PVdF with its unique technology cultivated through years of R&D on fluoromaterials and offers the lineup of "NEOFLON VT" series incorporating a new binder that imparts functionalities, including improved electrode flexibility, higher density, and gelation prevention. In addition, other binders including those for solid electrolytes and dry process are under development.
The new binder is considered to be a very promising material for a PVdF binder that can add new value while maintaining basic performance as a binder. For more information on this binder, please visit Daikin Industries’ product information page for battery materials.
RELATED ARTICLES

Serial column
11/2024
[Battery materials] Vol.3 Dry process for lithium-ion batteries
The market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the third column of the series is “Dry process for lithium-ion batteries.”

Serial column
08/2022
[Battery materials] Vol.1 Lithium-ion batteries and fluoromaterials
The market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the first column of the series is “Lithium-ion batteries and fluoromaterials.”



