
![]()
Serial column
08/2022
[Battery materials] Vol.1 Lithium-ion batteries and fluoromaterials
Takanori Suzuki
Suzuki Material Technology and Consulting Co., Ltd.
[Serial column] In this column, the market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the first column of the series is “Lithium-ion batteries and fluoromaterials.”
【Serial column : Battery materials】
Vol.01 Lithium-ion batteries and fluoromaterials
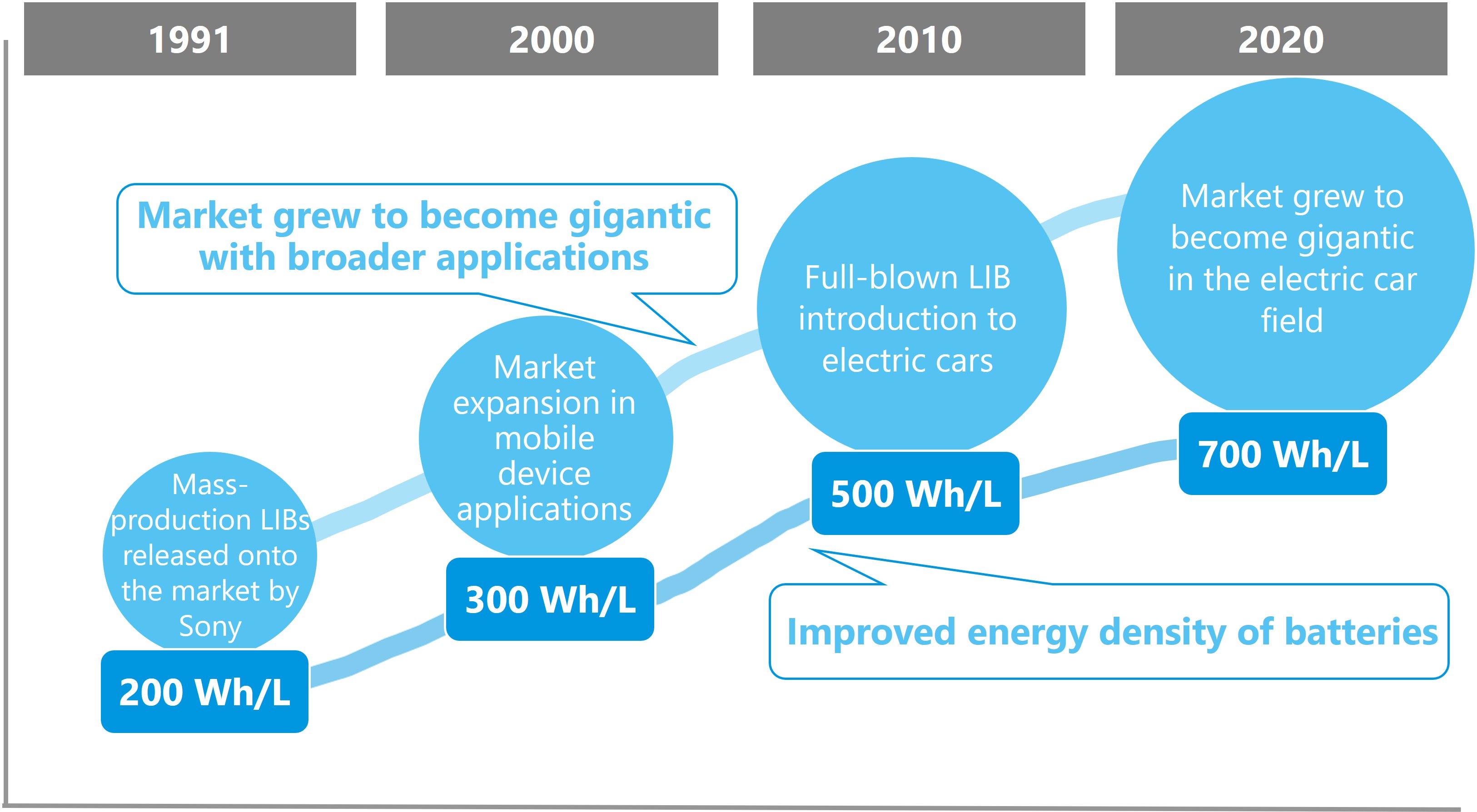
1. History of lithium-ion batteries
Lithium-ion batteries, which sprang from a fundamental view established by Dr. Akira Yoshino in 1985, have now grown into a gigantic market that continues to expand by finding extensive applications in such fields as communications, mobile units, and the environment. It goes without saying that Dr. Yoshino won the 2019 Nobel Prize in chemistry for this meritorious achievement.
Sony was the first manufacturer to release them onto the market in 1991. When lithium-ion batteries made their presence known to us, they came to be found in notebook computers, mobile phones, video cameras, digital cameras, and other mobile devices. The most advantageous characteristics of lithium-ion batteries are that they are small, lightweight, and high capacity. Without lithium-ion batteries, the mobile phone or smartphone market would have been very different.
In recent years, they have been present in broader applications such as automotive (EV, HEV, PHEV, etc. or xEV), energy storage, industrial devices, aircrafts, and trains, and in addition, they have been upsized. Their 30 years of history can be rephrased as a history of improvements to capacity and output density, reductions in cost, and maintenance of or enhancements to safety.
2. Trends in lithium-ion battery development
The characteristics that batteries are still expected to have are high capacity, high output, lightness, safety, and inexpensiveness. Especially, lithium-ion batteries, which people have high hopes for as “high-capacity batteries,” have come to need to meet requirements for high capacity with the highest priority. However, this is not the case where manufacturers did not care about other kinds of performance and proceeded with development while balancing out the required characteristics and at the same time increasing the capacity, and this is how today’s performance has been acquired.
Developments have taken place in material, design, and production perspectives independent from each other. The material perspective, which is required for such a development that allows many indirect materials to contribute to enhancing battery performance in addition to the development of the four major materials (cathode, anode, electrolyte, separator), have overcome all of these challenges.
Figure.1 History of lithium-ion batteries
2-1. Cathode
The development of cathodes pursues greater battery capacities by increasing the amount of lithium contained in the active material and by increasing the voltage. At the same time, the design process pursues cost reduction and stable material supply with the reduced use of cobalt and nickel, which are rare metals, and with the use of easy-to-get materials only.
2-2. Electrolyte
Studies on electrolytes have been conducted in combination with the improvement of withstand voltage to accommodate the cathode’s voltage, which has grown higher, as well as the development and selection of additives for the stable operation of the anode and the selection of solvents. Lithium-ion batteries are burdened with the big problem that they can burn, and this is attributable to the combustible organic solvent used as the electrolyte. Making electrolytes noncombustible is an enormous theme; and the solution coming up as an extension of this theme is solid-state batteries, which use a solid electrolyte.
2-3. Separator
The separator is very important material to ensure safety. Yet, it does not contribute to the role of storing electricity. Therefore, there is always a demand to make it as thin as possible while ensuring safety. It can be said that the successful development of a separator depends on meeting both these safety and thinness requirements. As another requirement, the separator needs to resist the cathode’s voltage, which has grown higher.
2-4. Anode
In order to gain higher capacity, the manufacturers of anodes are developing various methods, such as adding metal oxides like silicon, tin, and using the lithium metal itself as the anode.
2-5. Binder
Polyvinylidene fluoride (PVDF) materials have long been used for cathode binders since the advent of lithium-ion batteries. Although some improvements, including the introduction of high polymerization and functional groups, have been added, the use of PVDF materials as principal materials has still remained the same.
2-6. Conductive agent
As conductive agents, carbon black materials have been used for many years. However, against the background of recent years’ cost reductions, a multi-wall or single-wall CNT are now being released onto the market. A vapor deposition carbon existed in the past as well, but it seems to be a big breakthrough that we are now seeing some utilized as general conductive agents. CNTs are often supplied as dispersions.
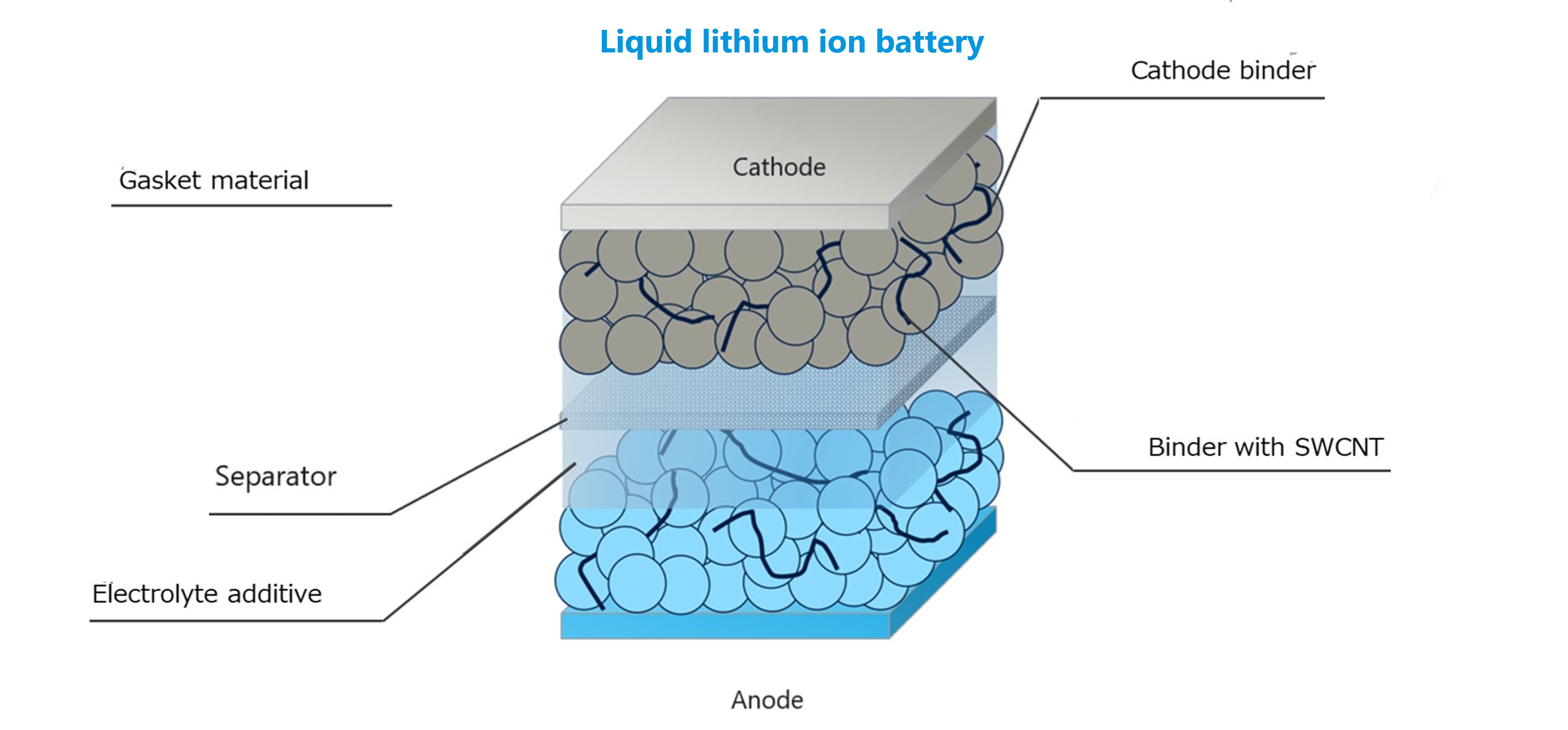
3. Material development by Daikin
Armed with experience gained over many years in fluorochemicals, Daikin is rolling out materials for lithium-ion batteries, such as binders, electrolyte additives and solvents, CNT-combined binder dispersions and gasket materials, and thereby has been contributing to performance improvement and safety assurance for lithium-ion batteries.
Figure.2 Daikin's battery materials
3-1. Relationship between fluorochemicals and lithium-ion batteries
Fluorine compounds and fluoropolymers are generally known for their high chemical stability, and many of these fluorine materials are used in lithium-ion batteries. Fluorine was a behind-the-scenes key player so indispensable that lithium-ion battery materials - especially electrolytes and binders - would not have helped to realize high-performance batteries if it had not been used.
Lithium-ion batteries can reach up to 4.6 V. This is due to the cathode, whose potential is very high. What other materials are there that could keep a stable performance under such an oxidizing condition? There are few except for fluorine compounds. Especially, electrolytes and binders, which lie in the cathode and are exposed to a strong oxidizing condition, must have extremely high stability.
3-2. Binder
Daikin’s product lineup includes NEOFLON VT-475, which is a new binder additive improved from PVDF thanks to Daikin’s own technology to impart functionalities, including improved electrode flexibility, higher density, and gelation prevention of slurry. In addition, other binders such as for solid state electrolytes and for those dry process are under development.
3-3. CNT dispersion
Daikin is proceeding with the development of NEOFLON VTD-475N, which is a binder-containing conductive agent dispersion combining a single-wall CNT and a binder elaborated based on Daikin’s binder. The market has high hopes for it as a product that can impart excellent conductivity to electrodes and also bring the binder performance of Daikin to batteries.
3-4. Electrolyte (additive)
Daikin is also developing a fluorine electrolyte additive designed to be used in electrolytes in order to assist their functionalities. This is another excellent fluorine material that maintains both controlled reactivity and stability when in a battery and thereby contributes to enhancing battery performance.
3-5. Gasket material
Gaskets are used as the seals around the terminals of prismatic lithium-ion batteries. Cylindrical cells also use gaskets to insulate and seal the cathode terminal and the can. These gaskets, which serve as terminal-can insulators and come into direct contact with the organic electrolyte in the can, are required to be chemically stable and resist pressing creep at the same time.
Meeting these required characteristics, Daikin’s fluoropolymer NEOFLON PFA is getting good reviews as a gasket material.
This material development that is possible only by Daikin, which has been dedicated to fluorochemistry over many years, delivers leading-edge technology to improve the performance of lithium-ion batteries and can play a major role in environmental conservation. I have great expectations that the company will continue contributing to society through fluoromaterials development.
RELATED ARTICLES

Serial column
11/2024
[Battery materials] Vol.3 Dry process for lithium-ion batteries
The market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the third column of the series is “Dry process for lithium-ion batteries.”

Serial column
10/2022
[Battery materials] Vol.2 Binder for lithium-ion batteries
The market trends and development movements of battery materials are featured by Takanori Suzuki, who has been engaged in the development of lithium-ion battery materials for many years and is currently a consultant for battery materials at Suzuki Material Technology and Consulting Co., Ltd. The theme of the second column of the series is “Binder for lithium-ion batteries.”



